Spectrophotometry
Fundamental
Light is a photon stream that moves at high speed and is also an electromagnetic wave with wavelength and frequency characteristics. The energy of a photon is proportional to the frequency and inversely proportional to the wavelength. The visible light is called visible light. Visible light only accounts for a very narrow portion of the electromagnetic spectrum (400 nm to 760 nm). Different wavelengths of visible light have different colors. Light with a wavelength greater than 760 nm is called infrared. Light having a wavelength of less than 400 nm is called ultraviolet light.
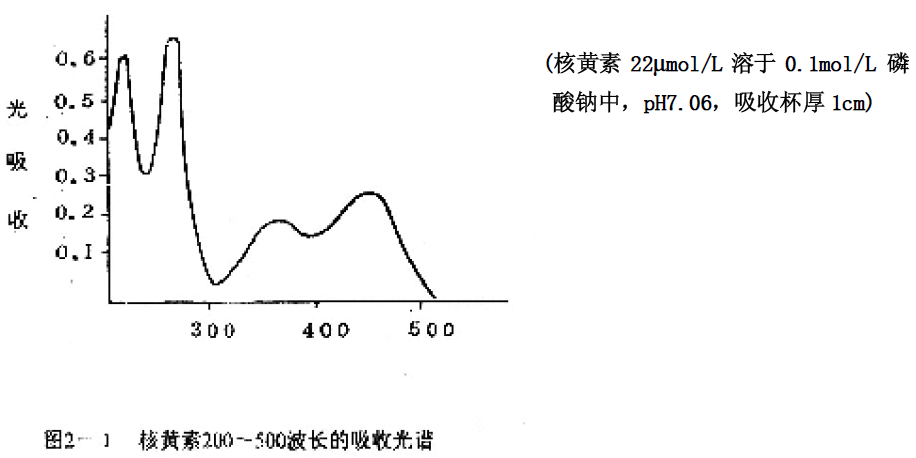
When a bundle of white light passes through a colored solution, light having a certain wavelength is selectively absorbed by the solution. Different substances have different absorption abilities for light of different wavelengths due to their different molecular structures, so each substance has its specific absorption spectrum. The determination of the absorption spectrum can be used to identify a variety of different materials. For example, riboflavin appears yellow because it absorbs only the blue light range in visible light and measures its absorption peak at 450 nm (Figure 2-1). It also has two absorption peaks in the ultraviolet range. They are 260 nm and 370 nm, respectively.
Spectrophotometry is often used to determine the concentration of light absorbing material present in a solution. The theoretical basis is the Lambert-Beer law.
(1) Lambert's Law
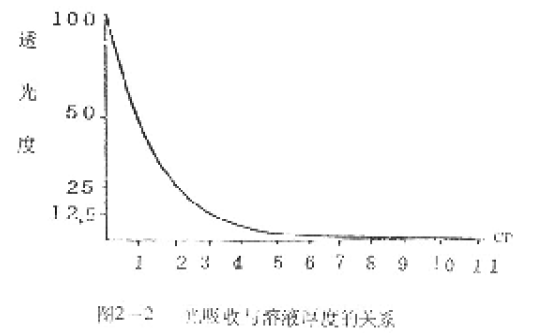
When a bundle of monochromatic light passes through a solution, the intensity of the light is attenuated because the solution absorbs a portion of the light energy. If the concentration of the solution is constant, the greater the thickness of the solution, the more pronounced the decrease in light intensity (Figure 2-2).
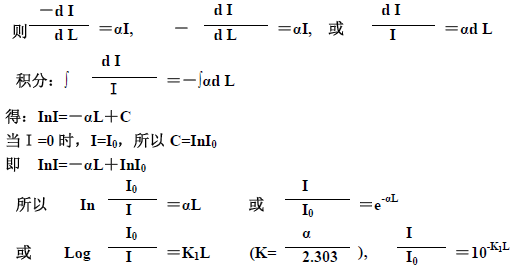
If I0 represents the incident light intensity, I represents the intensity of the light passing through the solution, and L represents the thickness of the solution.
(2) Beer's Law
When a bundle of monochromatic light passes through a solution, if the thickness of the solution is constant, the higher the concentration of the solution, the more pronounced the intensity of the light is, which is similar to the above law. The relationship between the two can be expressed as follows:

Where C represents the concentration of the solution. K2 is a constant that is affected by the wavelength of the light, the nature of the solution, and the thickness of the solution. The relationship between the light intensity expressed by the above formula and the solution concentration is called Beer's law. Although all solutions are in accordance with Lange's law, not all solutions are in accordance with Beer's law. This is because some substances may change their color under different concentration conditions, that is, the wavelength of the absorbed light changes under different concentration conditions. The common reasons are as follows:
1. Some colored substances may dissociate into corresponding ions in solution, and the color of the ions is different from the color of the molecules, causing errors in Beer's law.
2. Some substances can form complexes at higher concentrations, and the complex changes the absorption spectrum. For example, cobalt chloride is rosy in a dilute solution and blue in a concentrated solution.
3. Hydrogen ion concentration and electrolytes can also cause changes in the color of some colored substances, and these changes may also cause errors in Beer's law.
(3) Lang Bo - Beer's Law and Its Application
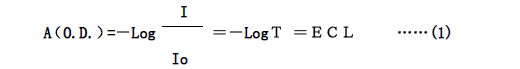
Generally, the ratio of light intensity (I) and incident light (Io) after passing through the solution is called transmittance (T), and -Log is expressed by absorbance (A) to reflect the light absorption of the solution. Happening. Sometimes it is also expressed by the optical density (OD). Then the relationship between them is as follows
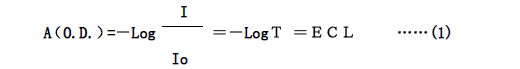
Where E is the extinction coefficient, which indicates the ability of the substance to absorb light, and its value varies depending on the type of material and the wavelength of light.
From equation (1), it is known that for the same substance and monochromatic light of the same wavelength (the extinction coefficient is constant), the absorbance of the solution is proportional to the concentration of the solution.
![]()
If C2 is the concentration of the standard solution, the concentration of the solution to be tested can be obtained according to the measured absorbance value according to formula (2).
In practice, for the sake of simplicity, it is often not necessary to make a standard tube for each sample to be tested. Instead, a series of standard tubes of different concentrations are measured in advance, and then the absorbance is plotted against the standard concentration to obtain a standard curve. After the absorbance of the substance, the corresponding concentration value can be found on the standard curve.
It can be known from the formula (1) that if the extinction coefficient of a substance to be tested and the thickness of the solution are known, the concentration of the solution to be tested can also be estimated from the absorbance. There are two common representations of the extinction coefficient:
1. Percent extinction coefficient (E1% 1 cm); extinction coefficient expressed as a percentage concentration. The percent extinction coefficient is equal to the absorbance value at a concentration of 1% and a liquid layer thickness of 1 cm.
2. Mercury extinction coefficient (ε); extinction coefficient expressed in molar concentration. The molar extinction coefficient is equal to the absorbance value of the solution concentration of 1 molar and the liquid layer thickness of 1 cm.
The formula for calculating the concentration using the extinction coefficient is:
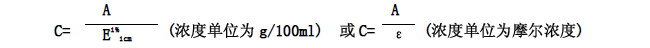
Shanghai Chuangsai Technology has excellent performance, interleukin cytokines, fetal bovine serum, electrophoresis equipment scientific instruments, raw material drug standards, chemical reagents, cell culture consumables, Shanghai Chuangsai, mass products special promotions, welcome to inquire!
Inflatable Toy Animal,Inflatable Water Toy Sea Animal,Crocodile Design Inflatable Water Toy,Summer Inflatable Toy Shark For Kids
J&TZ CO.,LTD , https://www.inflatable-toy.com