In recent years, neoprene adhesives have been widely used in automobiles, footwear, construction, furniture, and decorative industries, and their application range and usage have increased dramatically. However, the neoprene is easily decrystallized at temperatures above 50°C, and the cohesive force is significantly reduced. When the temperature is higher than 70°C, the adhesive strength is significantly reduced. Therefore, it is not suitable for use at higher temperatures, which greatly limits and affects its application and promotion. In order to improve the heat resistance of neoprene adhesives, some scholars have adopted carboxylated neoprene rubber, added chelating resins, room-temperature crosslinking agents, heated vulcanization accelerators, isocyanate-containing polyurethanes, and silane coupling agents. The degree of cross-linking of the molecular chains, and to a certain extent, increase the heat-resistant temperature.
This article summarizes the above research methods, and discusses the influence of the type of chloroprene rubber, chelating resin, and heating vulcanization accelerator on the heat resistance of the neoprene adhesive; the use of neoprene rubber and carboxylated neoprene rubber to obtain a bond. Good performance, high heat resistance neoprene adhesive, and has been successfully applied to industrial production.
1 Experimental section
1.1 Raw materials
Neoprene, A-30, A-90, A-120, Japan Electrochemical Corporation. Carboxylated chloroprene rubber, 510L, Japan Toyo Soda Corporation. P-tert-butyl phenolic resin, FRJ-551, softening point 110°C, Schenectady Chemical Company, USA. Tackifier, terpene phenolic resin, T803, softening point 145°C, Xiamen Arakawa Chemical Company. Active magnesium oxide, Shanghai Dunhuang Chemical Plant; active zinc oxide, Shanghai Jinghua Chemical Plant. Accelerator NA-22 (ethylene thiourea), accelerator CA (N, N'-diphenyl thiourea), Shandong Yicheng Aikesite Chemical Co., Ltd. Antioxidant, BHT (2,6-di-tert-butyl-p-cresol), Shanghai Xiangyang Chemical Plant. Catalyst, homemade.
1.2 Experimental Procedure
In a 1000 ml four-necked flask equipped with a dropping funnel, a reflux condenser, a thermometer, and a stirrer, 435 g of a mixed solvent was first added (volume ratio: toluene:n-hexane:ethyl acetate=1:1:1). Then take 100 parts of chloroprene rubber, 4 parts of active magnesium oxide, 4 parts of active zinc oxide, and 2 parts of antioxidant BHT. Mix and cool and chop on the mixer. Take 110g of crumb film and put it into a prescribed amount of mixed solvent. In a four-necked flask, stir and dissolve at room temperature for about 8 to 12 hours to prepare a neoprene adhesive with a solid content of 20%.
1.3 Performance Test
1.3.1 Determination of Viscosity of Viscosity According to the provisions of GB/T 2794-1995, the viscosity of the neoprene adhesive (pa.s/25°C) is measured with Brookfriel Company's LVF type viscometer.
1.3.2 Determination of bonding strength According to HG/T2493-1993 regulations, cotton canvas/cotton canvas sample strips were prepared, and the bonding strength (N/cm) was measured with a Guangzhou XL-50A tensile testing machine.
1.3.3 Determination of heat aging resistance According to HG/T 2493-1993 regulations, the bonding strength (N/cm) of the samples after 8 days at 80°C and 100°C was measured.
2 Results and Discussion
2.1 Effect of neoprene
Bonded neoprene rubbers A-30, A-90, A-120, carboxylated polychloroprene rubber 510L and mixed neoprene (A-120, 510L halves) were prepared according to the above experimental procedure. For 20% neoprene adhesive, add 30% neoprene T80 resin and test its adhesive strength at 25°C, 40°C, 60°C, 80°C, 100°C, as shown in Figure 1.
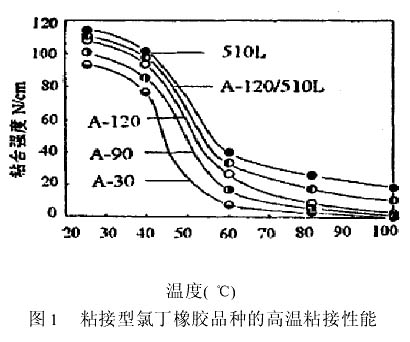
From the bond strength-temperature curve in Fig. 1, it can be seen that at 25°C, the carboxylated neoprene 510L of Japan Toyo Soda Co., Ltd. and the A series (A-120, A-90, Nippon Electric Chemical Co., Ltd.) were used. A-30) Adhesives prepared from adhesive chloroprene rubber and mixed neoprene (A-120/510L=1/1) with adhesive strength of 90 N/cm or more; at 40°C, adhesive strength is 77N/cm or more; and when the temperature rises above 60°C, the bond strength begins to drop drastically. The adhesive prepared from the A-series adhesive chloroprene rubber has a bond strength of 26N/cm or less at 60°C, 80 Decrease to 10N/cm or less at °C, and 4N/cm or less at 100°C. Adhesive prepared from carboxylated chloroprene rubber 510L reduces the adhesive strength to 40N/cm at 60°C. The adhesive strength decreases at 80°C. At 28 N/cm, the bond strength dropped to 20 N/cm at 100°C. Adhesive prepared from mixed neoprene (A-120/510L=1/1), the adhesive strength is reduced to 33N/cm at 60°C, the adhesive strength is reduced to 19N/cm at 80°C, and the adhesiveness at 100°C The intensity drops to 12N/cm. It can be seen that as the temperature rises, the adhesive strength of the adhesive gradually decreases. This is because the neoprene adhesive is based on the crystallizing effect of neoprene rubber to produce adhesive force, and it is usually easy to desolvate above 50° C., which leads to significant cohesive force. Reduced, decreased adhesion.
As can also be seen from Figure 1, the adhesive prepared from carboxylated chloroprene rubber 510L, at each temperature, the adhesive strength is greater than the adhesive prepared from the A series of adhesive chloroprene rubber, mixed by neoprene species (A -120/510L = 1/1) Adhesive prepared at each temperature with a bond strength between 510L and Adhesive made from A-120; and in the A-series adhesive neoprene In the rubber-made adhesive, the adhesive strength of A-120 at each temperature was greater than that of A-90 and A-30, and the adhesive strength of A-90 at each temperature was greater than that of A-30. This is due to the fact that carboxyl groups in carboxylated neoprene can form ionically crosslinks with metal oxides (such as MgO, ZnO), resulting in a rapid increase in adhesive strength, especially at high temperature, [7]; and A-120 molecular weight Larger than A-90, A-30, stronger cohesion, higher viscosity flow temperature, better heat resistance. [8] It can be seen that it is best to use carboxylated chloroprene rubber 510L to prepare a good heat-resistance neoprene adhesive, but correspondingly increase the cost of raw materials; mixed neoprene species (A-120/510L=1/1) The cost is reduced and the heat resistance is also very good and more suitable.
2.2 Effect of Chelating Resins
10 parts of magnesia, pt-butyl phenol resin FRJ-551 variable, 100 parts of non-polar mixed solvent (such as toluene, n-hexane, cyclohexane, etc.), 1 part of catalyst, stirring at about 25°C. For 16 hours, the precipitated material is filtered off after standing to obtain a chelated resin syrup. A blend ratio of neoprene rubber A-120 and carboxylated polychloroprene rubber 510L was 1:1. A neoprene adhesive with a solid content of 20% was prepared according to the above experimental procedure, and was prepared from different amounts of p-tert-butyl phenolic resin. The amount of chelating resin syrup used was 100 parts of chloroprene rubber. The amount of p-tert-butyl phenolic resin was 0, 15, 30, 45, 60, 75, 90, and 105 parts, respectively. Its adhesive performance, results shown in Figure 2.

From Figure 2, it can be seen that as the amount of p-tert-butylphenolic resin increases, the adhesive strength at each temperature first increases gradually and then gradually decreases: when the amount of resin reaches 45 parts, the adhesive strength at 25°C reaches a maximum value of 130N. /cm; When the amount of the resin reaches 60 parts, the high-temperature adhesive strength reaches the maximum (the maximum values ​​of the adhesive strength at 80°C and 100°C are 30 N/cm and 24 N/cm). This is due to the fact that the melting point of the chelate formed between magnesia and p-tert-butyl phenolic resin reaches 250°C. After addition of neoprene, the double bond of neoprene can be vulcanized and the hydroxymethyl group on the resin is chelated. It can form coupling with the carboxyl group of carboxylated chloroprene rubber, and further improve the adhesive strength and heat resistance; but the amount is too much, leading to film brittleness, reducing the adhesive strength [10]. And with the increase of the amount of resin, the possibility of layering glue gradually increased. This is because when the resin is not added, the surface of the metal oxide particles is adsorbed by the molecular weight of the neoprene molecules to form a thick protective film, which prevents the aggregation of the metal oxide and does not cause delamination; and after the addition of the resin, the polarity The strong low-molecular-weight resin replaces the molecular position of the neoprene so that the particles are sufficiently close to each other, and the particles interact with each other and eventually agglomerate and settle. At the same time, with the occurrence of the resin modification reaction, there will be a 2,6-dihydroxy 4-tert-butylphenol by-product, which can react with magnesium oxide and zinc oxide, resulting in the separation of the liquid phase and the occurrence of delamination. Taking into account various factors, the amount of p-tert-butylphenolic resin in the chelating resin syrup is preferably 50 parts.
Single Side Breast Pump
Zhejiang Carebao Co., Ltd , https://www.carebao.com